
Re: Want To Verify My Calculations
Dear anil0208,
Is the magnesium sulphate hydrous or anhydrous?
MgSO4 or MgSO4+7H2O
the material density is 2660 kgs/m3 for anhydrous and 1678 kgs/m3 fot hydrous. (Wikipedia)
That makes a difference for the suspension velocity of the 2 mm particles.
success
teus ■
Teus
Re: Want To Verify My Calculations
Dear anil0208
This is rahul well iwas going thru ur mail
can i have the soft copy of design parameter practised by u
regards
rahul ■
Thank You For Your Response, Sir
Originally posted by Teus Tuinenburg
Dear anil0208,
Is the magnesium sulphate hydrous or anhydrous?
MgSO4 or MgSO4+7H2O
the material density is 2660 kgs/m3 for anhydrous and 1678 kgs/m3 fot hydrous. (Wikipedia)
That makes a difference for the suspension velocity of the 2 mm particles.
success
teus
MY MATERIAL IS ANHYDROUS. PLEASE GIVE SOME SUGGESTIONS. ■
Thank You For Your Response, Sir
iI HAVE ATTACHED DESIGN PARAMETERS.
SO, PLEASE CHECK THE RESULTS AND GIVE SUGGESTIONS.
Attachments
■
Re: Want To Verify My Calculations
Dear anil0208,
In your project, you limit the pressure at 0.2 bar.
This 0.2 bar could be far less than the empty pipe line (only air) pressure drop.
That should mean that the product should have a negative product loss factor (to create pressure instead of decreasing pressure) , which is impossible.
Did you calculate the empty pipeline pressure and if so, what is the result of that calculation?
have a nice day
teus ■
Teus
Re: Want To Verify My Calculations
MY AIR ONLY PRESSURE DROP IS 0.0455 bar. ■
Re: Want To Verify My Calculations
Dear anil0208,
Base don your calculation, I calculated back the product loss factor for my calculation program.
The calculated product loss factor appeared to be approx.10 times higher than for “comparable” products.
Any calculation is as good as the input data are (belonging to the calculation method).
Those data must be obtained from experiments and tests.
The following table is just a reproduction of your calculations and does not reflect anything else.
I suggest that a field test with a bulk truck is executed and the derived data to be converted in product loss factors that can be used in your calculations.
anil0208 anil d 12-31-2007
Pressure discharge MgSO4
Convey length = 132 m
Nu of bends= 3
Pump vol = .21 + .21 m^3/s
q-convey = 0.21 m^3/s
Dia begin = 128.2 m Dia end = 128.2 m
Pipevolume = 1.7 m^3
------------Pipeline
Press.-------Cap-------SLR-----v-begin------- v-end-----kWh/ton-----res.time
0.200 -------1.8 -----2.00 --------16.5 ---------- 18.8-------5.59--------- 26.62
0.190--------1.7------2.00 -------16.6------------ 18.8 ----- 5.75-------- 25.40
0.180 --------1.6-----1.90---------16.8------------18.8 ------ 5.94 ------- 24.16
0.170--------1.5 -----1.80 -------16.9 -----------18.8--------6.17--------22.89
0.160---------1.4-----1.60 --------17.1 ----------18.7------- 6.45 ------ 21.59
0.150---------1.3-----1.50 -------- 17.2 ---------18.7 ------6.80 --------20.26
0.140---------1.2-----1.40 -------- 17.4-----------18.7------7.25---------18.89
0.130--------1.1 ------1.30 -------17.6 -----------18.7------7.86 -------- 17.47
0.120---------1.0-----1.10 ------- 17.8 -----------18.7------8.74---------15.99
0.110---------0.8-----0.90 ------ 18.0------------18.7------10.14--------14.44
0.100---------0.6-----0.70 -------18.2-------------18.7------12.89--------12.77
0.090---------0.3-----0.40 ------ 18.5-------------18.7------- 22.40-------11.03
Empty pipeline pressure ...= 687 mmWC
best regards
teus ■
Teus
Re: Want To Verify My Calculations
thank you very much that you have spent time for me, but i have calculated pressure loss by another methode from the chapter on "MULTIPHASE FLOW" in heat exchanger deisn handbook, and it is giving pressure drop with material is 8378 N/m^2 with the same parameters. it is little bit higher than air only pressure drop given by you.
can you tell me whether my approach to calculation is right or am i making mistake somewhere?
I wish you HAPPY NEW YEAR ■
Re: Want To Verify My Calculations
dear Anil0208
A recalculation, based on your last information requires a NEGATIVE product loss factor, which is impossible.
Check your calculation for the partial pressure drops:
p-acceleration .excluding product resistance
p-suspension
p-lifting
p-airfriction
p-productresistance
I strongly suggest a field test.
happy New Year
teus
anil0208 REMARK : 01-01-2008
*************** 10:12:17
PRODUKT :MgSO4
Pipeline capacity ........= 1.80 ton/hr
Convey Length = 132 m
System-pressure...........= 875. mmwC
Number of Bends = 3 -
Q-pump....................= 0.212 m^3/s
D-begin =128
D-end =128
Q-convey-pipe ............= 0.212 m^3/s
Outlet force .. = 47 N (dynamic)
loading-ratio ............= 2.09 - -
NO BOOSTER
Temp-MgSO4 = 48.8 / 40.4 Deg.C
Ambient temperature ......= 40.0 ‹C
T-out compressor = 63. Deg.C
Reynoldsnumber ..[ Re ]...= 1.32 --
Compr power .....= 8. kW
Total power .....= 8 kW
spec.energy-consumption...= 4.62 kWh/ton
backpressure at pipe-end..= 0. mmwC
p-accel.excl prod.resist..= 107. mmwC
p-suspension.............= 220. mmwC
p-lifting................= 29. mmwC
p-airfriction............= 622. mmwC
p-productresistance......= -192. mmwC ----(NEGATIVE PRODUCT LOSS FACTOR = NOT REAL)
p-intake productcolumn...= 0. mmwC
p-intake ................= 52. mmwC
p-nozzle ................= 67. mmwC
p-filter.................= 34„ mmwC
Empty pipe dp....= 687 mmWC
density product/air mix ..= 3.7 kg/m^3
Mass in pipeline.= 4 kg ■
Teus
Re: Want To Verify My Calculations
Sir, would you please tell me something about "product loss factor" because this is new term for me.
In my data temperature of Material at feeding point is 125 deg. C and air is also used at 125 deg. C to convey. ■
Re: Want To Verify My Calculations
dear Anil0208
When particles are conveyed through a pipeline by a gas flow, the particles are colliding with each other and against the wall.
Thereby they loose kinetic energy (velocity).
The gas flow reaccelerates the particle at cost of a pressure drop.
This pressure drop is depending of the energies, lost in these collisions.( non elastic, degradation) and relates only to the product conveying properties.
The rate of velocity losses are represented by a number ( or formula).
This number is named as the product loss factor and forms a part of the calculation algorithm.
I recalculated for a material temperature of 125 degrC and 40 degr C
When the material is 125 degrC then the capacity is (set) 1.8 tons/hr at 875 mmWC.
The air heats then up to 100 degrC.
Along the pipe wall, the mixture temperature is cooled by the pipewall against the ambient to approx 40 degrC.
When the material is then set at 40 degrC then the capacity is calculated at also 1.8 tons/hr
The conclusion is that the system is not sensitive for material temperatures, although we have to keep in mind that these calculations are done with an impossible energy source, the negative product loss factor.
Also other parts of the intended system play a role, such as filter pressure drop, product intake pressure drop, etc.
I am afraid that further mathematical investigations will lead to complete erratic confusions.
As long as we do not have reliable data for this product from field tests, we are guessing in the dark.
Sorry
teus ■
Teus
Re: Want To Verify My Calculations
Thank you sir
I will contact you if I will need further help. ■
Comparable Results
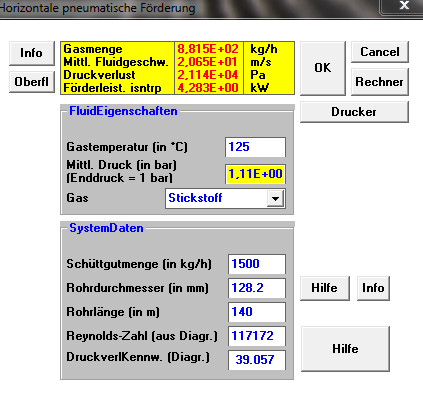
href="showthread.php?p=37889#post37889" rel="nofollow">
I AM ANIL, ENGG. STUDENT IN SVNIT-SURAT, INDIA. I AM HAVING PROJECT ON DESIGN OF PNEUMATIC CONVEYING SYSTEM. MY DUTY IS TO CONVEY MAGNESIUM SULPHATE AT RATE OF 1.5 TONNE/HR. I HAVE DONE CALCULATIONS USING YOUR BOOK "DESIGN GUIDE LINES FOR PNEUMATIC CONVEYING" ELSEVIER PUBLICATION. FOLLOWING DATA I HAVE CALCULATED. I WANT TO KNOW WHETHER ITS RIGHT OR WRONG BECAUSE MY CONVEYING TEMPERATURE LITTLE HIGH AND PARTILE DIAMETER IS ALSO 2 mm?
MATERIAL : MAGNESIUM SULPHATE,
PARTICLE DIAMETER : 2 mm
1. MATERIAL MASS FLOW RATE : 1.5 TONNE/HR
2. DIAMETER OF PIPE : 5" SHEDULE 40, ID=128.20 mm
3. PIPELINE LENGTH:
HORIZONTAL : 120 m
VERTICAL UP : 11.5 m
TWO 90 Deg. ELBOWS : D/d=10
4. ALLOWABLE PRESSURE DROP : 0.2 bar
5. BLOWER RATING : 0.3 bar
6. MIN. CONVEYING VELOCITY ASSUMED : 16 m/s
7. AIR TEMPERATURE : 125 deg C
Hi there,
I want to use this opportunity to compare the results of a high temperature conveying line design after the Book "Design Guide Lines For Pneumatic Conveying" (Elsevier Pblication) with the outcome of the calculation by my software. I calculated the case of minimum pressure drop in dilute phase, and as one can see, the results are practically identic.
ManfredH

href="https://forum.bulk-online.com/attachment.php?attachmentid=32297&d=1333633779" id="attachment32297" rel="Lightbox74167" target="blank">■




Want To Verify My Calculations
HELLO SIR, DR. DAVID MILLS,
I AM ANIL, ENGG. STUDENT IN SVNIT-SURAT, INDIA. I AM HAVING PROJECT ON DESIGN OF PNEUMATIC CONVEYING SYSTEM. MY DUTY IS TO CONVEY MAGNESIUM SULPHATE AT RATE OF 1.5 TONNE/HR. I HAVE DONE CALCULATIONS USING YOUR BOOK "DESIGN GUIDE LINES FOR PNEUMATIC CONVEYING" ELSEVIER PUBLICATION. FOLLOWING DATA I HAVE CALCULATED. I WANT TO KNOW WHETHER ITS RIGHT OR WRONG BECAUSE MY CONVEYING TEMPERATURE LITTLE HIGH AND PARTILE DIAMETER IS ALSO 2 mm?
MATERIAL : MAGNESIUM SULPHATE,
PARTICLE DIAMETER : 2 mm
1. MATERIAL MASS FLOW RATE : 1.5 TONNE/HR
2. DIAMETER OF PIPE : 5" SHEDULE 40, ID=128.20 mm
3. PIPELINE LENGTH:
HORIZONTAL : 120 m
VERTICAL UP : 11.5 m
TWO 90 Deg. ELBOWS : D/d=10
4. ALLOWABLE PRESSURE DROP : 0.2 bar
5. BLOWER RATING : 0.3 bar
6. MIN. CONVEYING VELOCITY ASSUMED : 16 m/s
7. AIR TEMPERATURE : 125 deg C ■